Publications
Publications
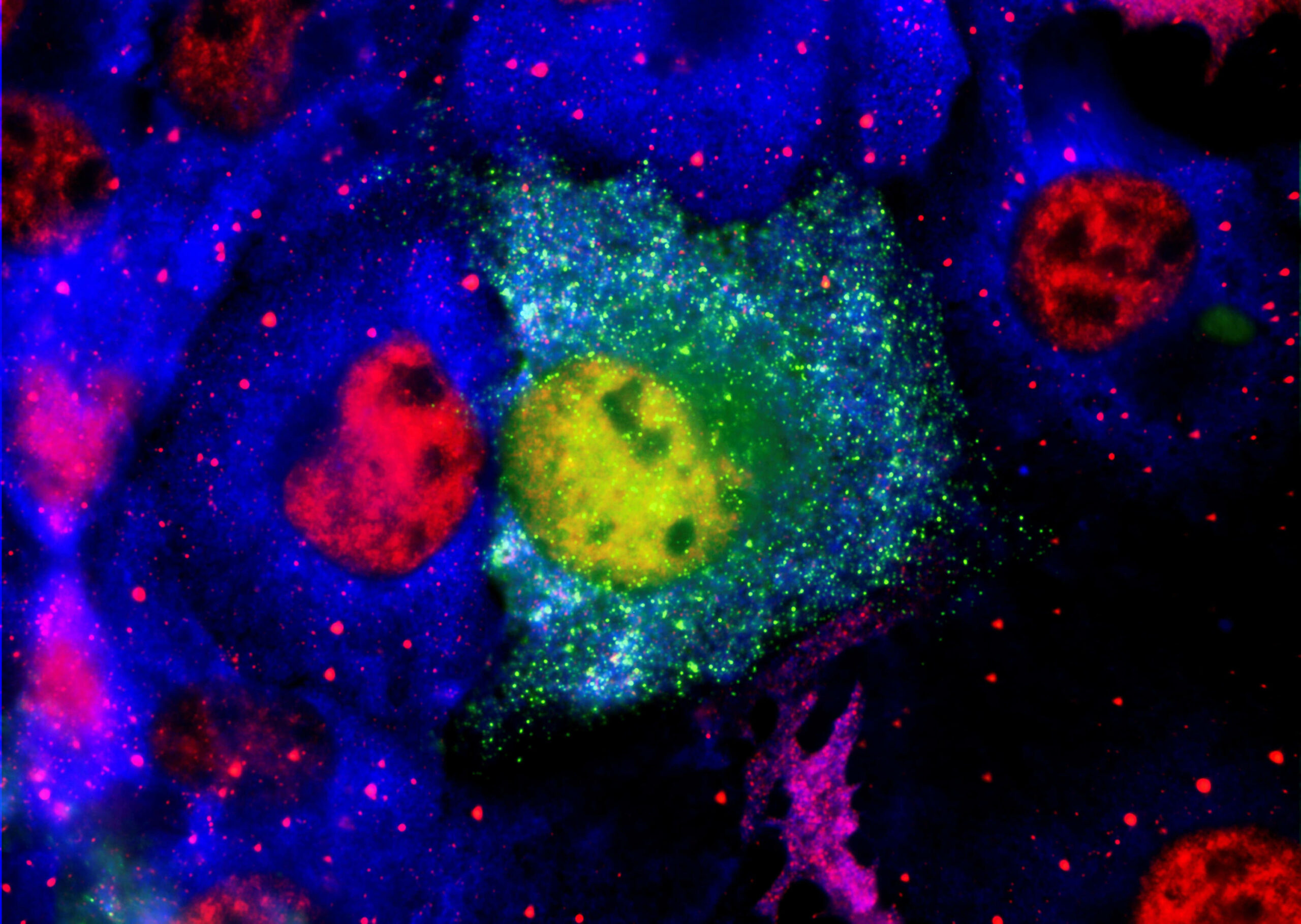
Viral Involvement in Alzheimer’s Disease
Abstract: Alzheimer’s disease (AD) is characterized by the presence of β-amyloid plaques (Aβ) and neurofibrillary tangles (NFTs) in the brain. The prevalence of the disease is increasing and is expected to reach 141 million cases by 2050. Despite the risk factors associated with the disease, there is no known causative agent for AD. Clinical trials with many drugs have failed over the years, and no therapeutic has been approved for AD. There is increasing evidence that pathogens are found in the brains of AD patients and controls, such as human herpes simplex virus-1 (HSV-1). Given the lack of a human model, the route for pathogen entry into the brain remains open for scrutiny and may include entry via a disturbed blood-brain barrier or the olfactory nasal route. Many factors can contribute to the pathogenicity of HSV-1, such as the ability of HSV-1 to remain latent, tau protein phosphorylation, increased accumulation of Aβ in vivo and in vitro, and repeated cycle of reactivation if immunocompromised. Intriguingly, valacyclovir, a widely used drug for the treatment of HSV-1 and HSV-2 infection, has shown patient improvement in cognition compared to controls in AD clinical studies. We discuss the potential role of HSV-1 in AD pathogenesis and argue for further studies to investigate this relationship.
Drug Repurposing: progress, Challenges and recommendations
Abstract Given the high attrition rates, substantial costs and slow pace of new drug discovery and development, repurposing of ‘old’ drugs to treat both common and rare diseases is increasingly becoming an attractive proposition because it involves the use of de‑risked compounds, with potentially lower overall development costs and shorter development timelines. Various data-driven and experimental approaches have been suggested for the identification of repurposable drug candidates; however, there are also major technological and regulatory challenges that need to be addressed. In this Review, we present approaches used for drug repurposing (also known as drug repositioning), discuss the challenges faced by the repurposing community and recommend innovative ways by which these challenges could be addressed to help realize the full potential of drug repurposing.
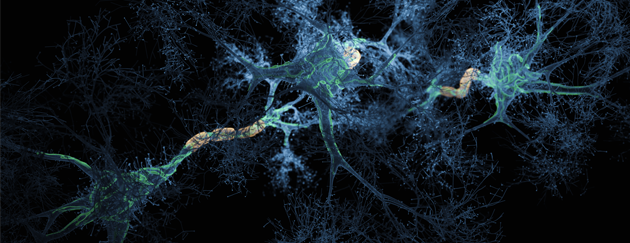
Differential Expression of AMPA Subunits Induced by NMDA Intrahippocampal Injection in Rats
Abstract Glutamate is involved in excitotoxic mechanisms by interacting with different receptors. Such interactions result in neuronal death associated with several neurodegenerative disorders of the central nervous system (CNS). The aim of this work was to study the time course of changes in the expression of GluR1 and GluR2 subunits of glutamate amino-acid-3-hydroxy-5-methyl-isoxazol-4-propionic acid (AMPA) receptors in rat hippocampus induced by NMDA intrahippocampal injection. Rats were submitted to stereotaxic surgery for NMDA or saline (control) microinjection into dorsal hippocampus and the parameters were evaluated 24 h, 1, 2, and 4 weeks after injection. The extension and efficacy of the NMDA-induced injury were evaluated by Morris water maze (MWM) behavioral test and Nissl staining. The expression of GluR1 and GluR2 receptors, glial fibrillary acidic protein (GFAP), and neuronal marker (NeuN) was analyzed by immunohistochemistry. It was observed the impairment of learning and memory functions, loss of neuronal cells, and glial proliferation in CA1 area of NMDA compared with control groups, confirming the injury efficacy. In addition, NMDA injection induced distinct changes in GluR1 and GluR2 expression over the time. In conclusion, such changes may be related to the complex mechanism triggered in response to NMDA injection resulting in a local injury and in the activation of neuronal plasticity.
Inhibition of protein aggregation and amyloid formation by small molecules
Abstract For decades, drug after drug has failed to slow the progression of Alzheimer’s disease in human trials. How compounds reducing fibril formation in vitro and toxicity in transgenic mice and flies bind to the Ab toxic oligomers, is unknown. This account reviews recent drugs mainly targeting Ab, how they were identified and report their successes from in vitro and in vivo experimental studies and their current status in clinical trials. We then focus on recent in vitro and simulation results on how inhibitors interact with Ab monomers and oligomers, highly desirable knowledge for predicting new efficient drugs. We conclude with a perspective on the future of the inhibition of amyloid formation by small molecules
The N‑Methylated Peptide SEN304 Powerfully Inhibits Aβ(1−42) Toxicity by Perturbing Oligomer Formation
Abstract Oligomeric forms of β-amyloid (Aβ) have potent neurotoxic activity and are the primary cause of neuronal injury and cell death in Alzheimer’s disease (AD). Compounds that perturb oligomer formation or structure may therefore be therapeutic for AD. We previously reported that D-[(chGly)-(Tyr)-(chGly)-(chGly)-(mLeu)]-NH2 (SEN304) is able to inhibit Aβ aggregation and toxicity,shown primarily by thioflavin T fluorescence and MTT (Kokkoni, N. et al. (2006) N-Methylated peptide inhibitors of β-amyloid aggregation and toxicity.Optimisation of inhibitor structure. Biochemistry 45, 9906−9918). Here we extensively characterize how SEN304 affects Aβ(1−42) aggregation and toxicity, using biophysical assays (thioflavin T, circular dichroism, SDS-PAGE, size exclusion chromatography, surface plasmon resonance, traveling wave ion mobility mass spectrometry, electron microscopy, ELISA),toxicity assays in cell culture (MTT and lactate dehydrogenase in human SH-SHY5Y cells, mouse neuronal cell death and synaptophysin) and long-term potentiation in a rat hippocampal brain slice. These data, with dose response curves, show that SEN304 is a powerful inhibitor of Aβ(1−42) toxicity, particularly effective at preventing Aβ inhibition of long-term potentiation. It can bind directly to Aβ(1−42), delay β-sheet formation and promote aggregation of toxic oligomers into a nontoxic form, with a different morphology that cannot bind thioflavin T. SEN304 appears to work by inducing aggregation, and hence removal, of Aβ oligomers. It is therefore a promising lead compound for Alzheimer’s disease.