Professor Sophie Jackson
Cambridge University

Specialist in Protein Mis-folding
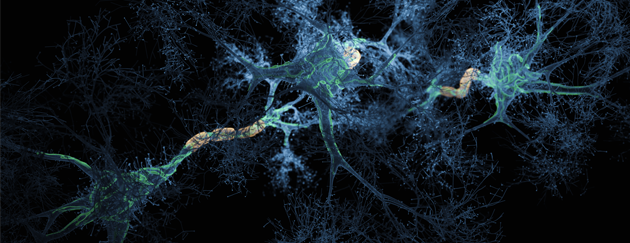
Sophie Jackson is professor of Chemical and Molecular Biology in the Yusuf Hamied Department of Chemistry, University of Cambridge Prof. Jackson’s is a world-leading scientist in the areas of protein folding and peptide aggregation and research at the interface of chemistry and biological sciences. Prof. Jackson’s experimental work covers diverse areas including biophysics, molecular biology (including protein engineering) and chemical biology. Recently she has studied protein misfolding and aggregation of amyloid β (Aβ) and α-synuclein (α-syn) that form amyloid fibrils in Alzheimer’s and Parkinson’s diseases, respectively, a process that is intimately linked to the diseases’ progression. In addition, her work has led to elucidating factors that affects physical stability of peptides that are important in developing therapeutics for disease.
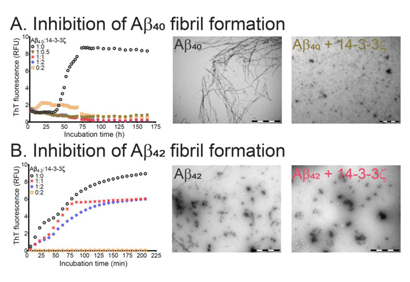
Prof Jackson publishes about inhibition of fibrils in the journal Molecules.
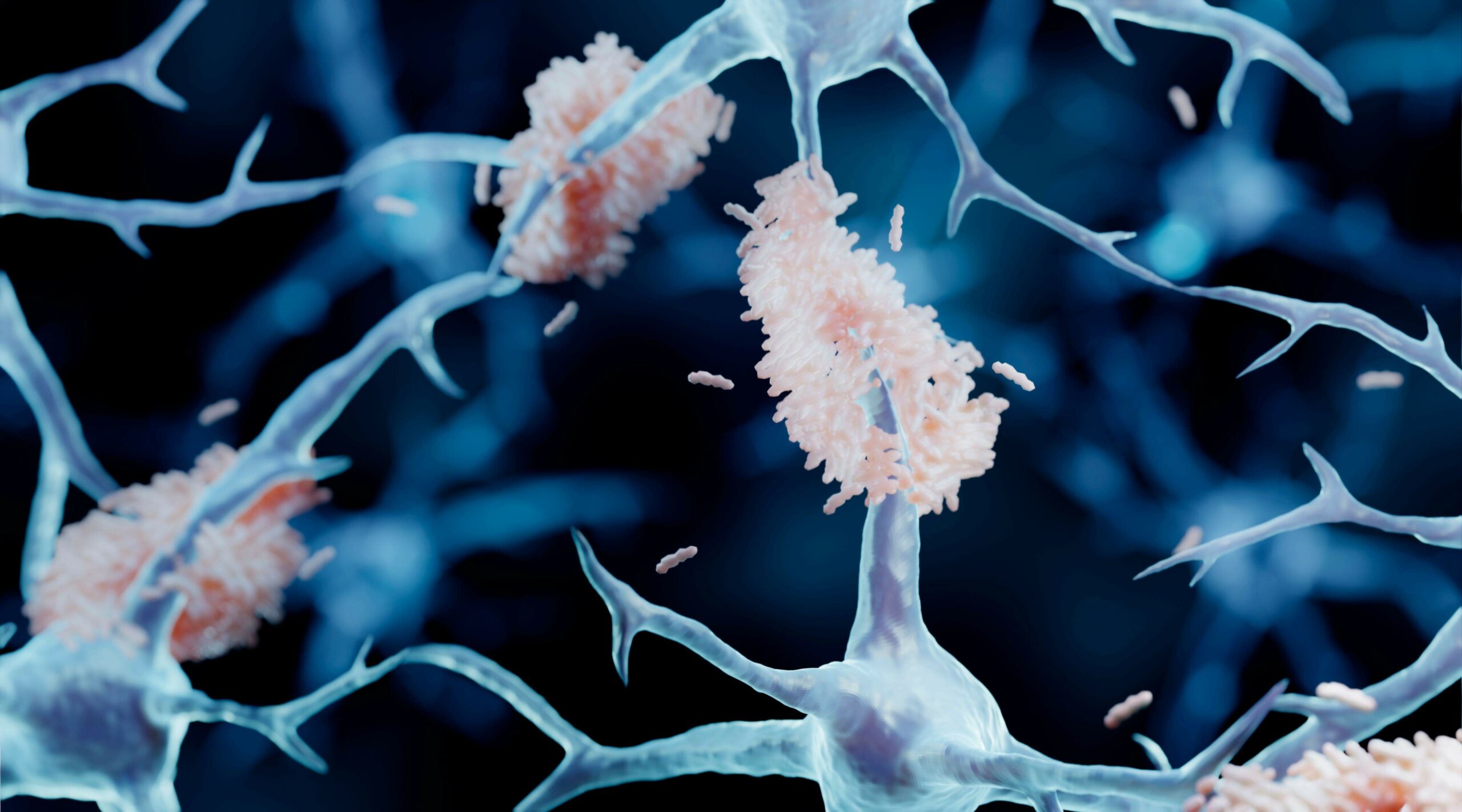
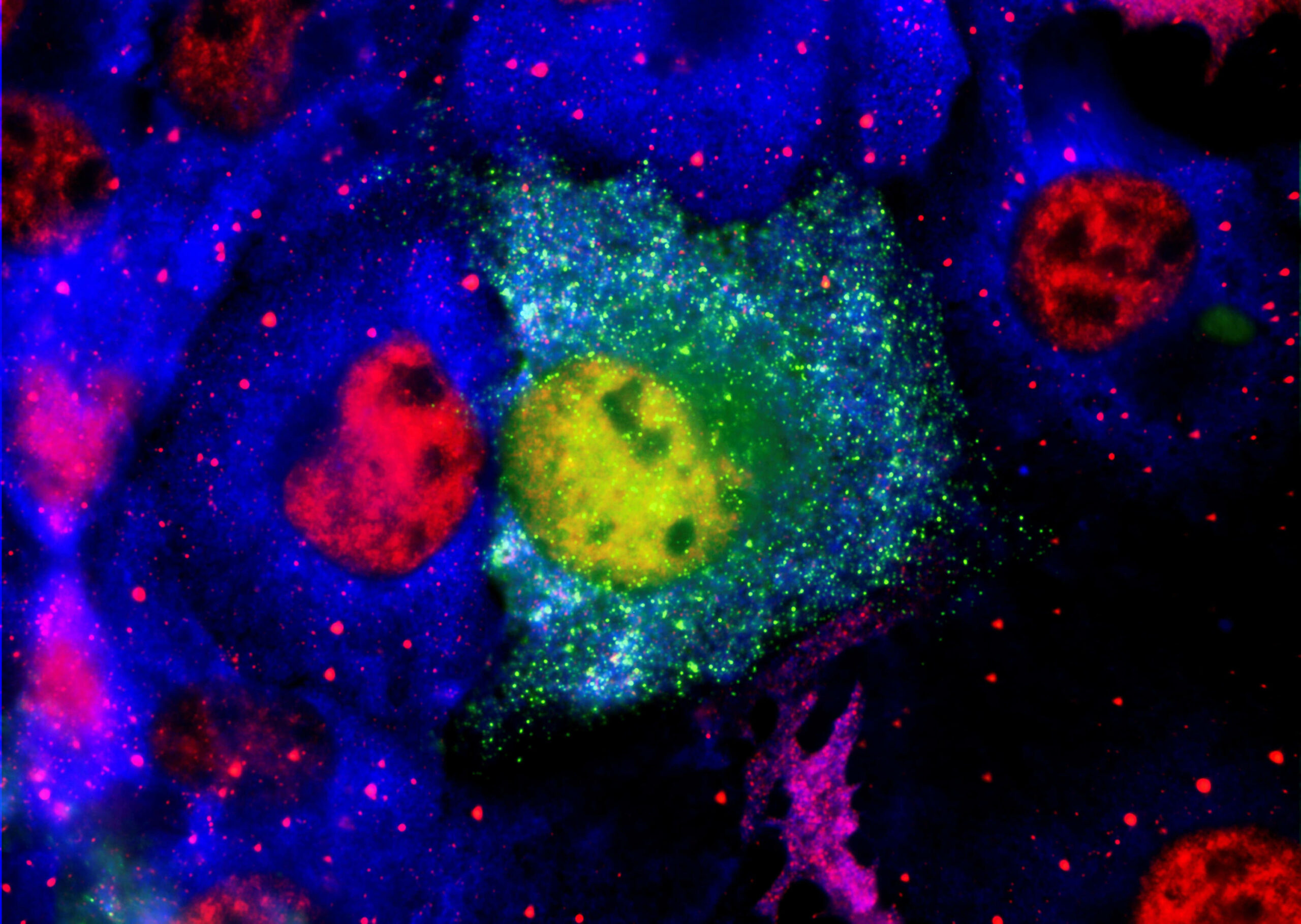
The article demonstrates that certain proteins that act as molecular chaperones can inhibit the formation of amyloid fibrils. The two major neurological diseases associated with protein aggregation and deposition are AD and PD . AD is characterized by the extracellular deposition of plaques containing mainly variants of the amyloid beta peptide (Aβ) in an amyloid fibrillar form, along with intracellular neurofibrillary tangles containing mainly the tau protein. The principal Aβ peptides are 40 and 42 amino acids in length. In PD, Lewy body deposits are the defining morphological intracellular feature; they are mainly comprised of the protein α-synuclein (α-syn), also deposited as amyloid fibrils. In AD and PD, other proteins are associated with these deposits, including sHsps and 14-3-3 proteins . Their presence may arise from the utilization by cells of their chaperone ability as an attempt to prevent the aggregation of Aβ and α-syn to form amyloid fibrils.
Prof. Jackson’s paper highlights the need for characterization of biotherapeutics.
'The number of biological therapeutic agents in the clinic and development pipeline has increased dramatically over the last decade and the number will undoubtedly continue to increase in the coming years. Despite this fact, there are considerable challenges in the development, production and formulation of such biologics particularly with respect to their physical stabilities…. The effects of sequence, concentration, pH, net charge, excipients, chemical degradation and modification, surfaces and interfaces, and impurities are all discussed. In addition, the effects of physical parameters such as pressure, temperature, agitation and lyophilization are described. We provide an overview of the structures of aggregates formed, as well as our current knowledge of the mechanisms for their formation.’

Prof Jackson talks about self-assembly processes which are extremely common in Nature.
These include unimolecular reactions like the folding of a polypeptide chain into its native, functional structure and RNA into unique three-dimensional conformations. However, self-assembly processes also occur where multiple molecules of the same type form higher-order functional aggregates. In some cases, these are functional species which play an important role in Biology, such as the aggregation of carotenoid pigments such as lutein into aggregates into the macula and retina in eyes where it has a photoprotective effect. In other cases, self-assembly processes are deleterious and associated with a large number of disease states, e.g. Amyloid-beta (Ab) peptide in Alzheimer's Disease. Some of the biological self-assembly systems our group will be described along with the experimental methods used to investigate them.