PK081
PK081
Therapeutic Drug
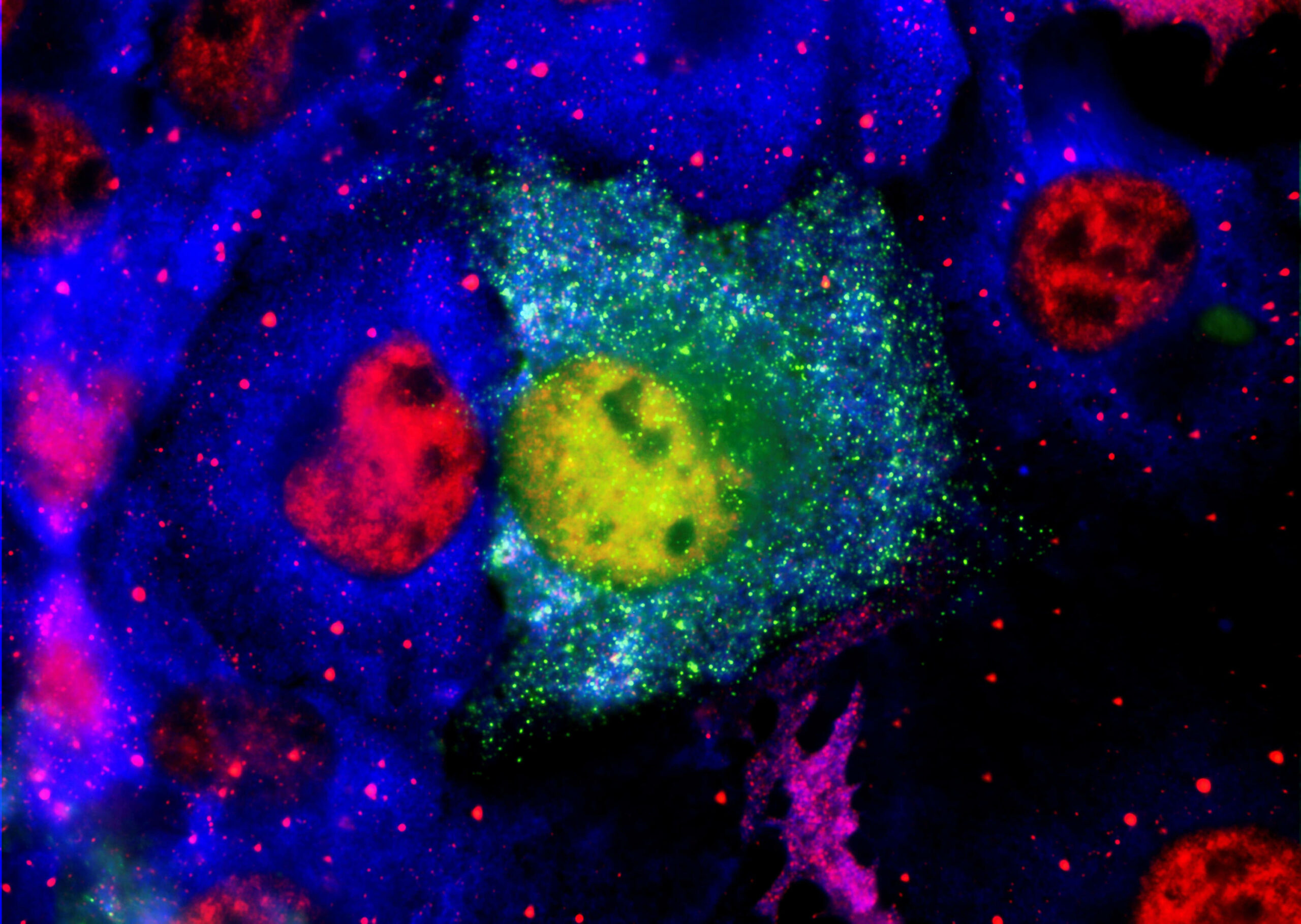
The anti-depressant drug used in PK051, belongs to the tricyclic family, which have been shown to prevent the aggregation of α-synuclein in Parkinson’s disease. PharmaKure is screening their compound library to match this with a drug that breaks down the aggregates for their PK081 candidate to treat Parkinson’s disease, Dementia with Lewy Bodies and MSA.
Summary
• Use of two drugs (an antidepressant and antipsychotic drug) for the dis-aggregation of toxic oligomeric proteins & other possible symptoms associated with Parkinsonism diseases, including Parkinson’s disease, Dementia with Lewy Bodies and MSA.
• The drug action is through dis-aggregation of previously formed toxic oligomers of α-synuclein proteins and prevention of aggregates forming.
• Expansion of a commercial formulation from a patient prospective as a combined single tablet, capsule or suspension aligned to the clinical program.
• A patent has been applied for to protect this formulation.
Status
Phase 2a Multiple Ascending Dose study commencing Q4 2022
Strength
• Seeking a 505(b) clinical pathway from FDA
• Seeking an advancement of Hybrid Scheme clinical pathway under Article 10 directive 2001/83/EC from EMA
• Expansion of formulation and biomarker may bring enhanced clinical outcomes
• Has the potential to become a prophylactic and/or first line of a cost effective treatment against early AD.