PK051
PK051
Therapeutic Drug
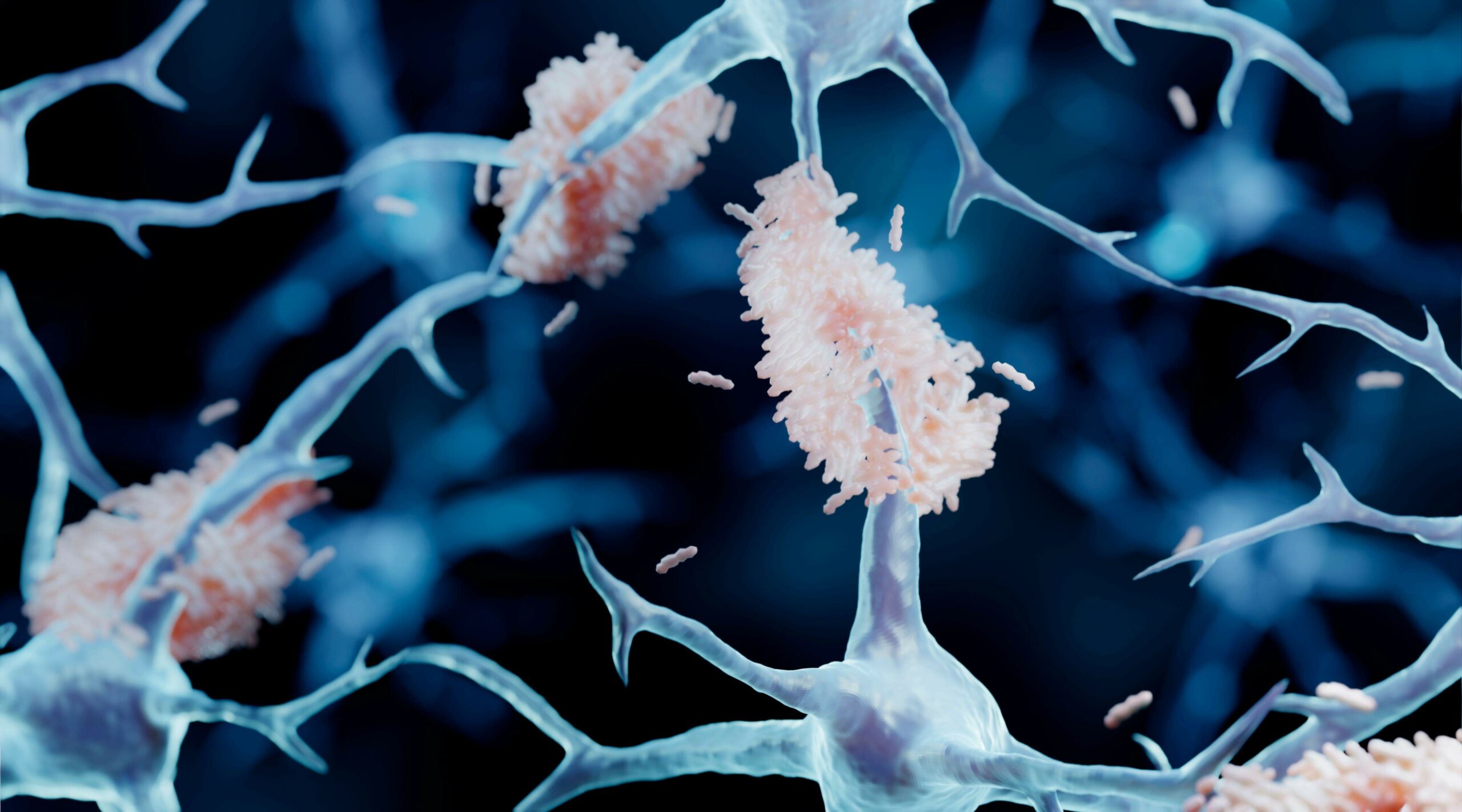
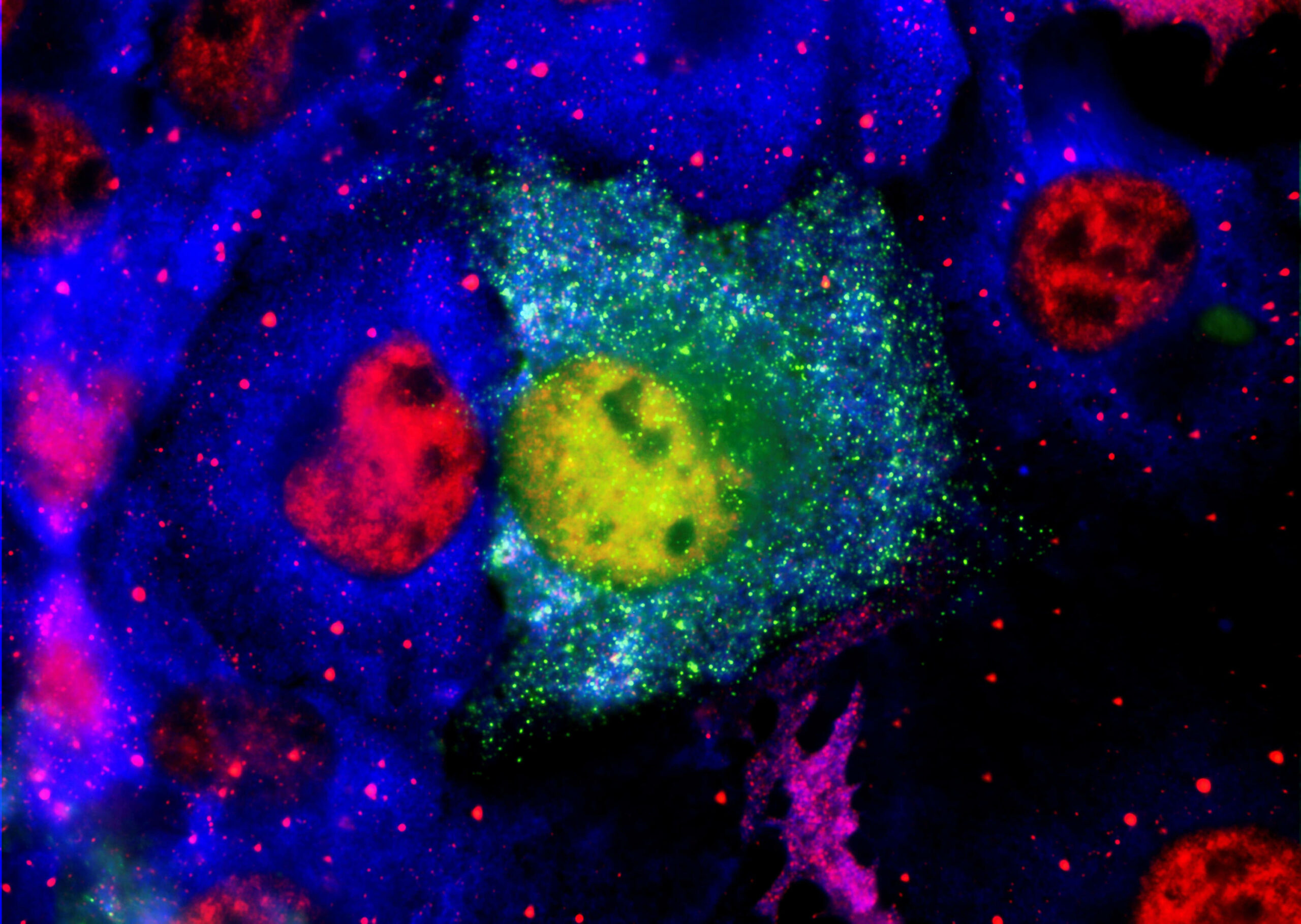
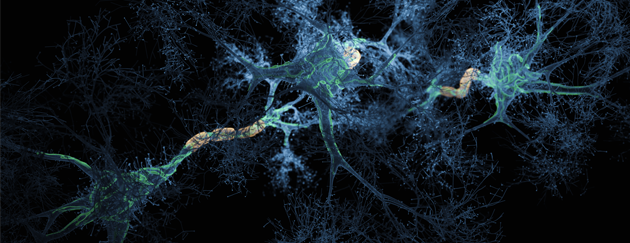
Aggregated amyloid-β and tau proteins form deposits (plaques) in the brain and other endpoints for early & mild stage patients diagnosed with Alzheimer’s disease.
PK051 a proprietary formulation of two existing drugs that in-vitro have been shown to break down aggregates of amyloid-β and prevent aggregate formation. In animal models treated mice did not develop plaques in the brain, whereas control mice did. In a rare human disease with a similar mechanism to brain pathology in Alzheimer’s disease, a compassionate use treatment saw reversal of disease symptoms for a year.
Summary
• Use of patented combination of two drugs (an antidepressant and antipsychotic) for the dis-aggregation of toxic oligomeric proteins & other possible symptoms associated with Alzheimer’s disease (AD)
• The drug action is through dis-aggregation of previously formed toxic oligomers of amyloid proteins and prevention of the released monomers acting as seeds to catalyse formation of new oligomers
• Expansion of a commercial formulation from a patient perspective as a combined single tablet, capsule or suspension aligned to the clinical program.
• Other potential indications include ‘brain fog’ associated with long COVID, post traumatic stress disorder
Status
Commencing Phase 2a Multiple Ascending Dose study commencing Q4 2022
Strength
• Seeking a 505(b) clinical pathway from FDA
• Seeking an advancement of Hybrid Scheme clinical pathway under Article 10 directive 2001/83/EC from EMA
• Expansion of formulation and biomarker may bring enhanced clinical outcomes
• Has the potential to become a prophylactic and/or first line of a cost effective treatment against early AD.