Dr. Farid Khan
CEO

Director and Co-Founder of PharmaKure
Dr. Farid Khan is a co-founder of PharmaKure Ltd. He has worked previously for GlaxoSmithKline as a drug discovery scientist and had academic positions in biotechnology at The Babraham Institute in Cambridge and at The University of Manchester. He also founded a CRO in biotech services which specialises in recombinant DNA technology; a drug repurposing company focused on Alzheimer’s disease; an artificial intelligence company focused on image based diagnosis of cancers; and a med-tech platform delivering medication adherence during clinical trials. He works with third party biotech and pharmaceutical companies undertaking biotech deal flows, including the licensing of drug assets. He has published papers in peer reviewed journals – including fast-tracking new indications for drugs for malaria. He has formed key interdisciplinary collaborations with industry, academic institutions and charities (such as the Welcome Trust) including stakeholders in the UK’s healthcare agenda. Dr Khan has previously received awards including ‘excellence in engineering/ science/ technology’ by the former Prime Minister David Cameron and an Alumni Achievement Award in science and engineering from the University of Salford. He has a PhD in protein engineering, a MSC in parasitology and a BSc in Chemistry. He is a senior Research Associate in Biotech and Enterprise at Peterhouse, The University of Cambridge.
Drug Repurposing in AD- Interview with Dr Farid Khan
Dr Farid Khan, CEO and Chair of PharmaKure has a background in assay development and HTS for drug discovery from GlaxoSmithKline. In this interview in ASSAY and Drug Development Technologies he discusses future prospects of drug repurposing.
‘Drug repurposing by its very nature is a cost-effective method of drug discovery because much of the safety aspect of the drug candidate is known. I foresee that failed drugs in terms of efficacy in AD may be further ‘‘repurposed in clinical trials’’ and that new biomarkers and lower clinical endpoints will become achievable… we believe the approach will at least delay the onset of this devastating disease.”
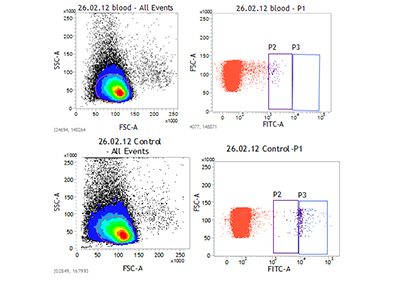
Drug Screening and Repurposing
Often current drugs can be fast-tracked as treatments for other diseases. Its not only the fact that previous safety allows the use of ‘repurposed drugs’ but also assays which are more relevant to human models are essential.
In the following collaboration with The University of Salford, we were able to screen FDA approved drugs in malaria using ‘ex-vivo’ infected human blood. The logic was if we could measure the ‘death’ of malarial parasites in human blood, then any active drugs which are orally administered would work. In effect we did not need to test the drugs in humans as blood was the perfect proxy. The positive control was artemisinin and new drugs were identified that had similar anti-malarial activities. One of these drugs was emitine, which is a drug used in the treatment of amebiasis.

Biomarker Assay Development and Protein Engineering
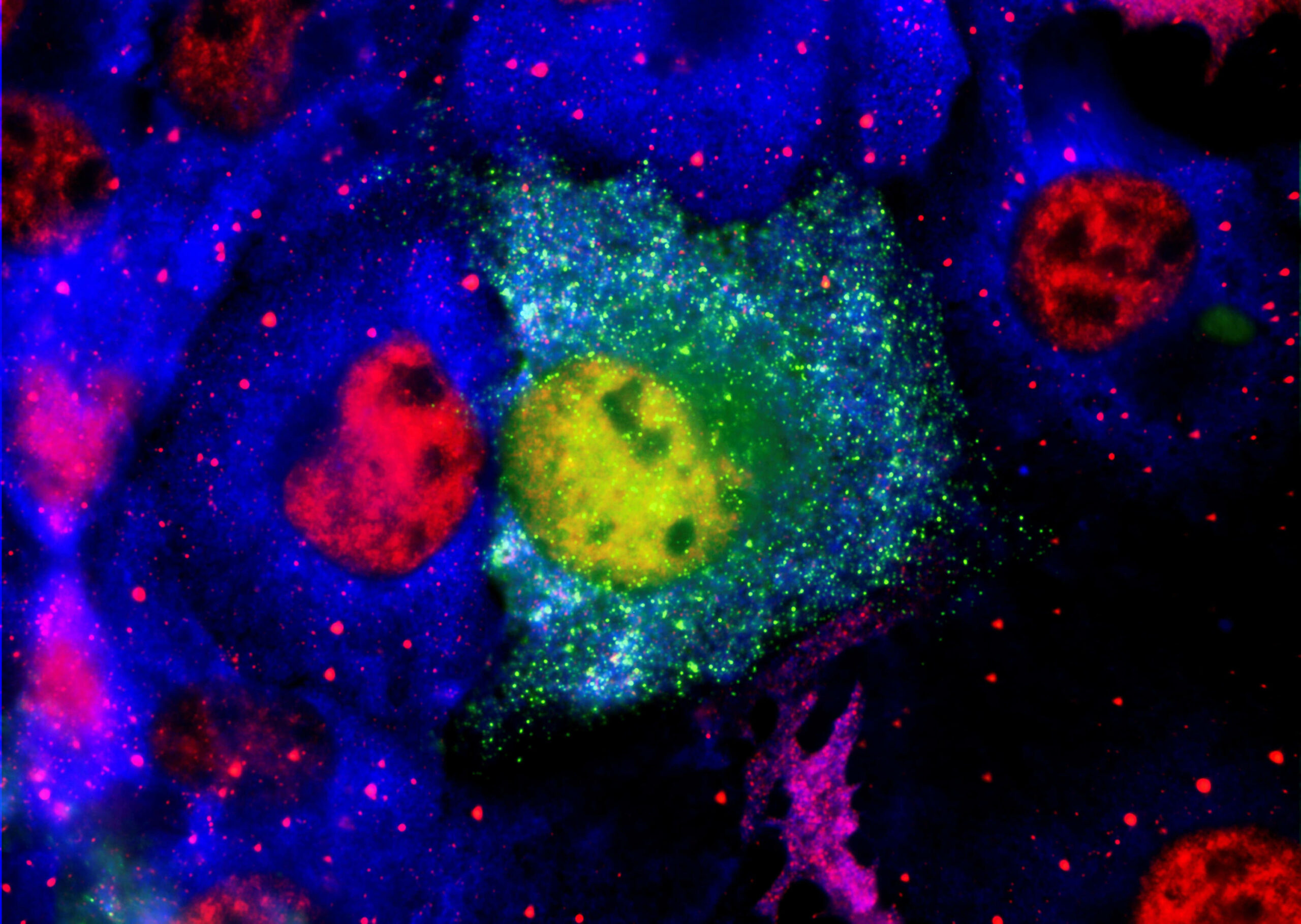
Alzheimer’s disease (AD) is a progressive neurodegenerative disease, characterized by β-amyloid (Aβ) plaques, neurofibrillary tangles, gliosis, oxidative stress, neuronal degeneration, and dementia. It was discovered twenty years ago that dysregulation of cellular Ca2+ leads to impaired cognition and increased susceptibility to AD and other neurodegenerative conditions. In particular Ca2+-dependent protein phosphatase calcineurin –a dependent transcription factor, nuclear factor of activated T cells (NFAT) has been implicated in AD and serves as a valid target for AD.
Dr. Khan and colleagues characterized novel engineered fluorescent proteins which allowed the development of both translocation and transcriptional assays. This dual cell-based assay format will enable the discovery of new drugs targeting the NFATc1A signal transduction pathway.