PharmaKure granted MHRA Clinical Trial Authorisation (CTA) for PK051 for the treatment of mild cognitive impairment due to Alzheimer’s Disease
Clinical Trial Authorisation application granted for combined drug PK051 targeting amyloid deposits associated with Alzheimer’s Disease
Manchester, United Kingdom, 4th January 2024 – PharmaKure, a clinical stage pharmaceutical company developing precision medicines for Alzheimer’s Disease and other neurodegenerative diseases, today announces that the UK Medicines and Healthcare Regulatory Agency (MHRA) has granted Clinical Trial Authorisation (CTA) for PharmaKure (or the “Company”) to commence a muti-ascending dose Phase 2a study to evaluate safety and tolerability of PK051 intended for the treatment of mild cognitive impairment (MCI).
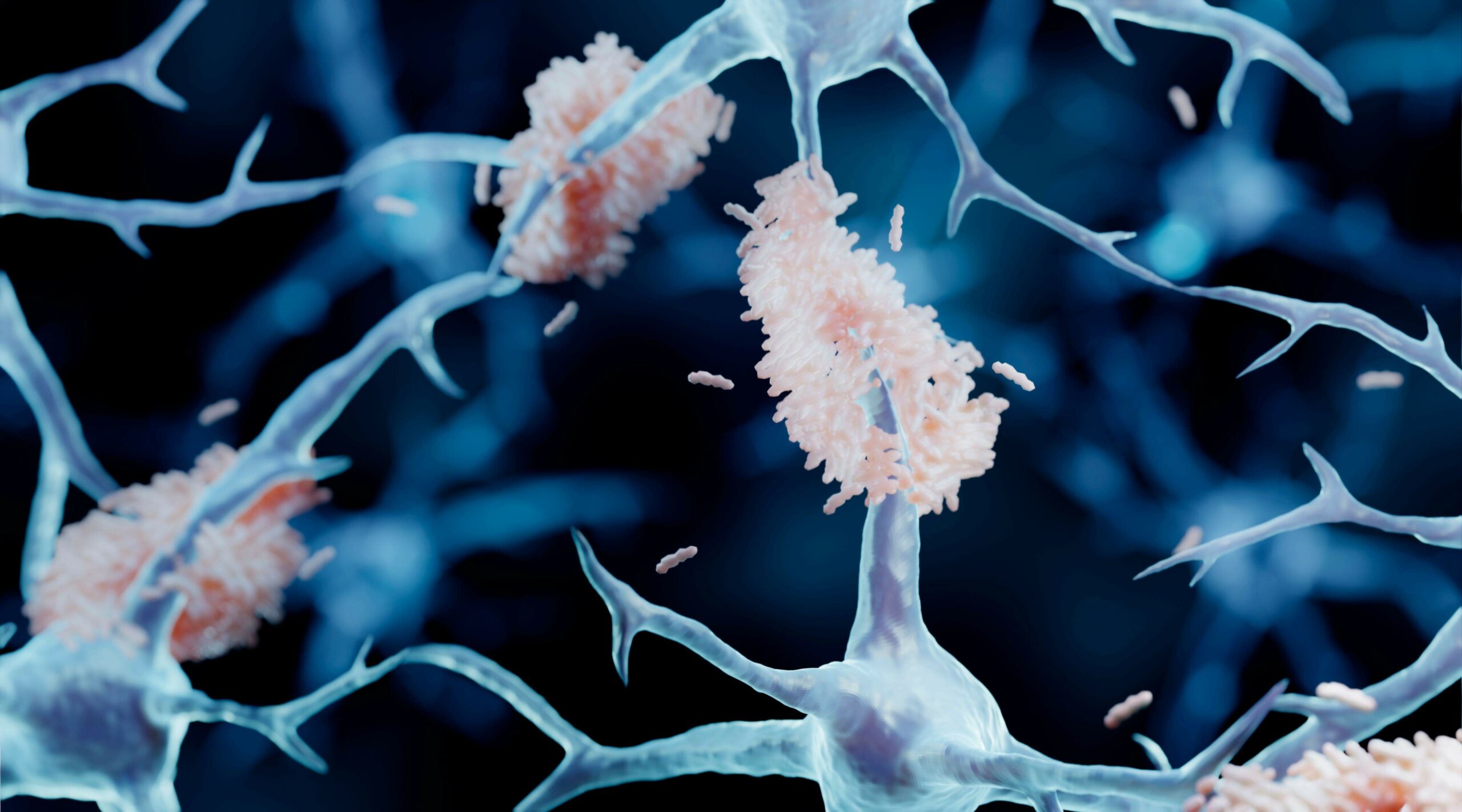
PK051 is an oral combined drug that targets disaggregation of amyloid-β proteins. There is increasing scientific acceptance that overproduction and/or deposition of amyloid-β is the initial event in Alzheimer’s Disease pathology.
“The MHRA authorisation marks a major step forward in our mission to develop PK051 as a disease modifying therapy for MCI due to Alzheimer’s Disease,” said Dr Farid Khan, CEO of PharmaKure. “This authorisation follows successful study results recently announced by the Company for a novel whole blood test to quantify Alzheimer’s Disease biomarkers. PharmaKure’s proprietary ALZmetrix™ blood test can identify blood-based biomarkers in patients with Alzheimer’s Disease to provide early warning of cognitive decline. Used as a companion diagnostic, this could enable treatments such as PK051 to be offered earlier to provide better population-based health outcomes.”
Dr Bob Smith, Chief Clinical Director, PharmaKure Limited, commented, “We are delighted to have approval to begin clinical testing of PK051. This Phase 2a study is intended to confirm safety, tolerability and to help us determine an appropriate dose for future efficacy studies. The trial will involve 40 patients with MCI due to Alzheimer’s Disease at a single site in the UK. The first patient is expected to be dosed in early to mid 2024, with preliminary clinical data emerging within 12 months of first dose.”
Editors’ notes
About Alzheimer’s Disease – Alzheimer’s disease is a fatal illness that causes progressive decline in memory and other aspects of cognition. Dementia due to Alzheimer’s is the most common form of dementia, accounting for 60 to 80 percent of all cases. Every three seconds someone in the world develops dementia. 850,000 people (UK) and 44 million people (worldwide) are currently suffering from Alzheimer’s or dementia related illnesses. In 2020, the total cost of care for people in the UK was £34.7 billion. Globally, the cost was $360 billion and by 2050 it could reach $1 trillion (source Alzheimer’s Research UK), unless we find a way to slow the disease.
About PharmaKure – PharmaKure is a UK based pharmaceutical company, spun out from the University of Manchester. The company is actively researching, developing, commercialising and repositioning repurposed drugs for the treatment of Alzheimer’s, Parkinson’s and other neurodegenerative diseases, as well as developing the ALZmetrixTM diagnostic.
For more information, contact [email protected], or see Pharmakure’s website www.pharmakure.com
Company Contacts
CEO: Dr Farid Khan – [email protected]
Head of R&D: Prof Andrew Doig – [email protected]
CCO: Dr Naz Bashir – [email protected]