PharmaKure Announces a Blood Biomarker Study
1st July 2022. PharmaKure is exited to announce a a blood biomarker study using their proprietary ALZmetrix® assay with Alzheimer’s disease (AD) patients. Currently AD is underdiagnosed because of the difficulties in obtaining an accurate diagnosis in the early stages of disease progression. A key factor in poor outcomes in AD trials is the difficulty in recruiting patients at the same progression of disease. The variation produces inconclusive data. The common feeling in the industry is that there are promising candidates, but the clinical trial data is not clean enough to prove this statistically.
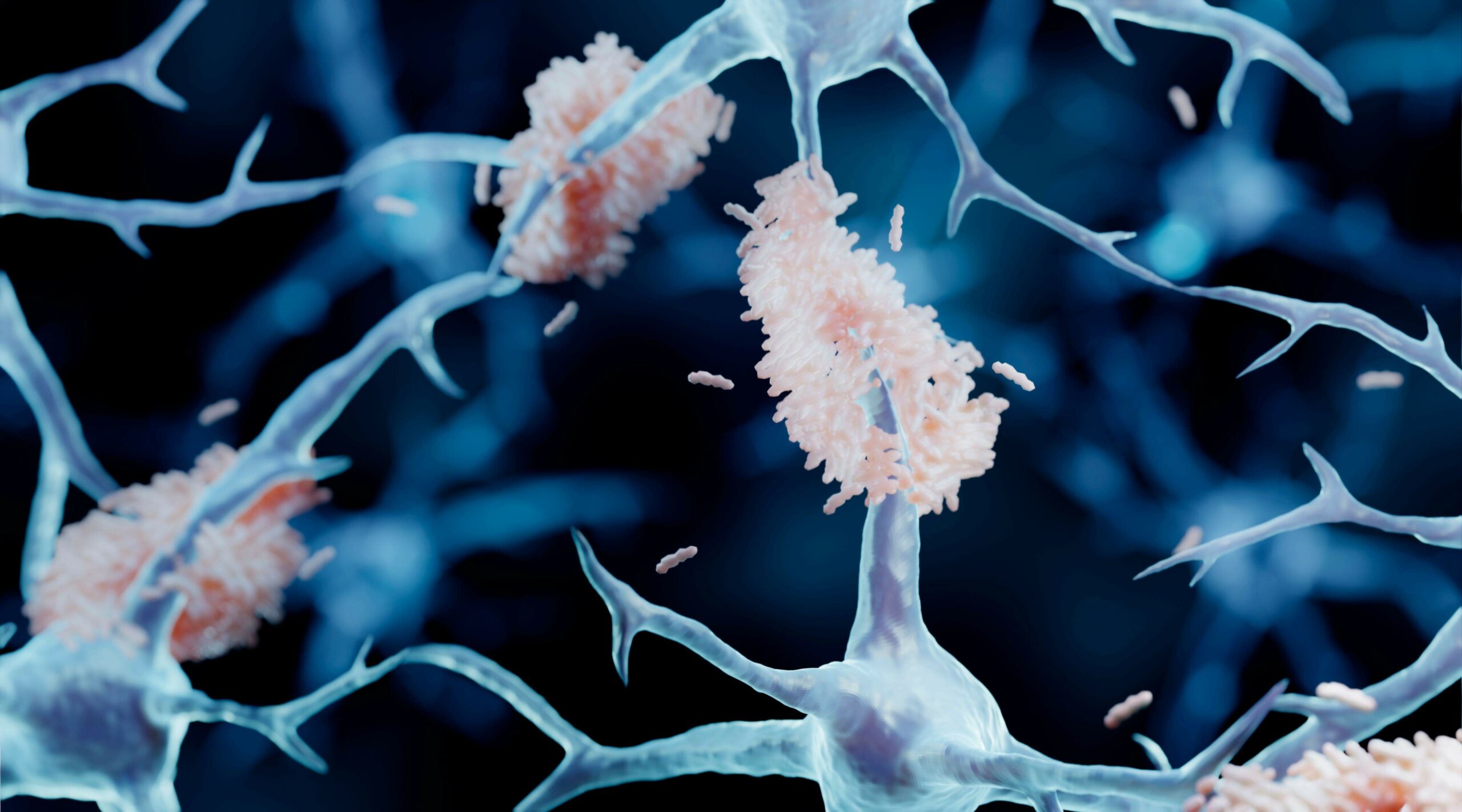
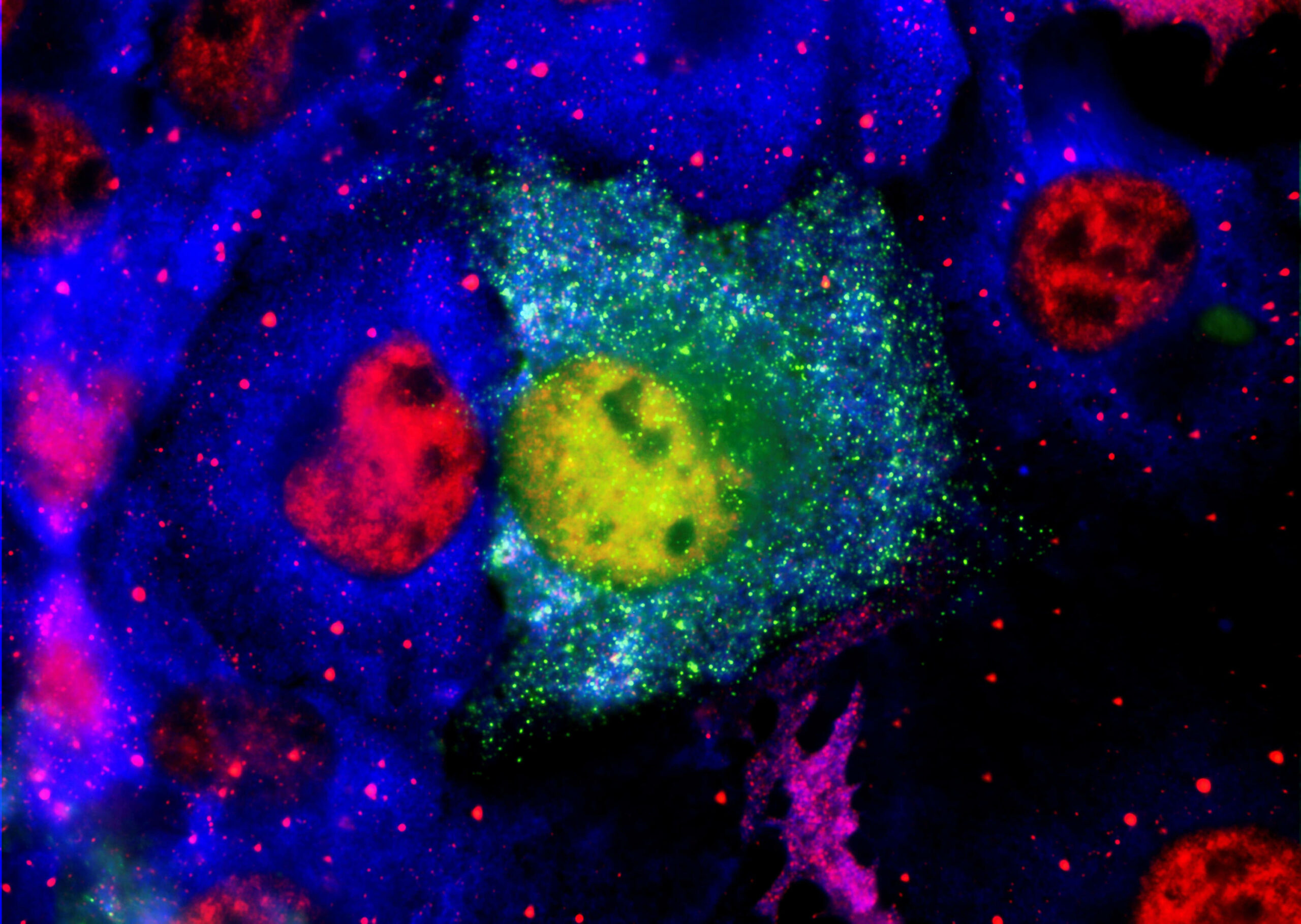
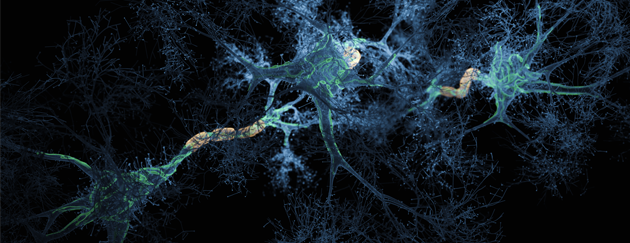
Alzheimer’s disease involves a misfolded protein called amyloid-ß. As this protein misfolds, it links to other proteins causing misfolding and they stick together building up into aggregates. The small soluble forms have been shown to be cytotoxic and cause the damage in Alzheimer’s disease. They are referred to as “oligomers” to differentiate them from larger insoluble aggregates that form the protein deposits or plaques in the brain.
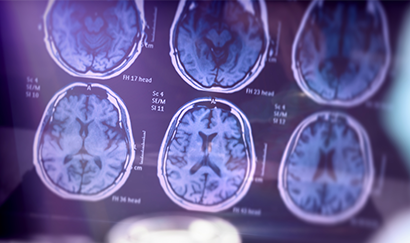
The primary objective of the Biomarker Study is to evaluate the relationship between aggregated biomarkers associated with AD including oligomers in the blood with historical PET scans and CSF findings.
ALZmetrix® measures oligomeric amyloid-ß levels in blood. A key breakthrough for PharmaKure was proving that these oligomers are present in blood andbound to blood cells.. By grouping patients with similar levels of oligomeric amyloid-ß as a recruitment criteria, PharmaKure expects to see clearer results for the trials on their lead candidate PK051.
This is not the entire story though. There are patients diagnosed with Alzheimer’s disease who have different presentation of plaques in the brain. In addition to cognition tests, Alzheimer’s diagnosis can be supported by brain scans that can visualise the amount of amyloid-ß plaque deposits. Some of these patients have Alzheimer’s symptoms, but are plaque negative. Perhaps they do not have Alzheimer’s or are a subgroup who respond differently to drug trials. We don’t know. This Study could allow to answer some these questions.