PharmaKure Completes the Acquisition of the CynapseDx Business with Financing involving the UK Government Future Fund
March 26th 2021. PharmaKure Limited (“PharmaKure” or “the Company”), a clinical stage biopharmaceutical company committed to accelerating the development of therapies for treating Alzheimer’s and rare neurodegenerative diseases, today announced it has acquired the business and assets of CynapseDx Ltd (‘CDX’), whose breakthrough biomarker technology will dramatically enhance and complement PharmaKure’s technology for the treatment of patients suffering from Alzheimer’s Disease and Related Dementias (ADRD).
Financing from the Future Fund together with Slievemara Limited supported the acquisition of CDX, which was executed as an asset purchase.
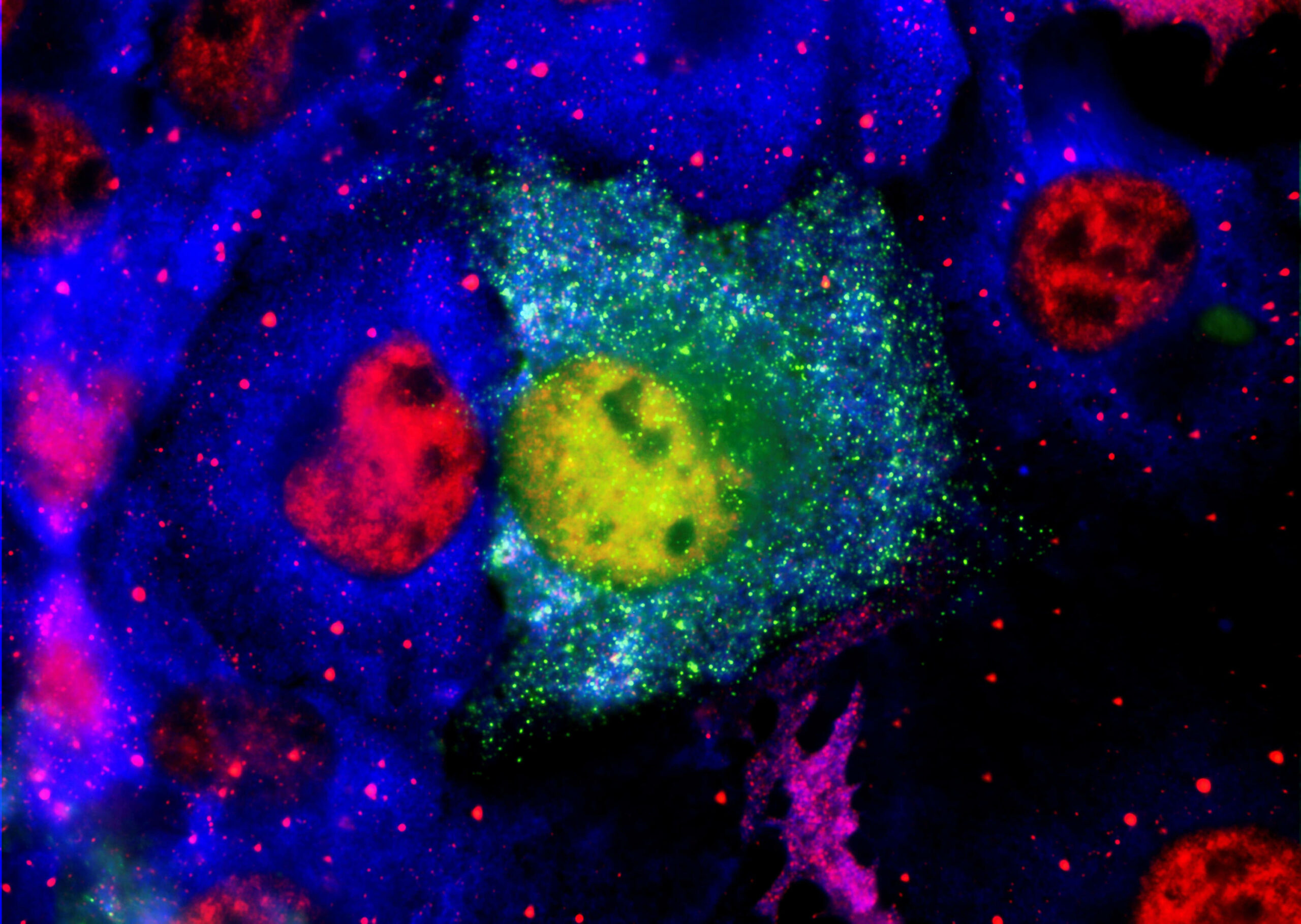
Part of CDX’s patent pending technology is a series of biomarker assays, which can uniquely determine the presence of the misfolded proteins, which are a cause of Alzheimer’s Disease. Their assays have been optimised to measure the misfolded forms of amyloid-β and α-synuclein in blood, rather than the more invasive procedure to test cerebral-spinal fluid, used by other investigators. Not only is this unique, but their work has demonstrated that both of these proteins are elevated in ADRD diseases, something that has been overlooked in other work. They have also demonstrated that the misfolded proteins are attached to blood cells and not free In the plasma surrounding the blood cells (a major reason other blood tests have failed as they only investigate plasma). This observation led to a proposed treatment based on a treatment that is routinely and widely used in the NHS to treat diseases with abnormal blood cells like Sickle Cell Anaemia. The treatment is called Apheresis and selectively removes a patient’s blood cells and replaces them with clean donor cells, reducing the reservoir of the toxic misfolded proteins in the body.
PharmaKure plans to use the unique biomarker tests created by CDX in a number of ways. Firstly, to screen its clinical trial drug candidates for their ability to interfere with the misfolded proteins to remove their toxic properties. Secondly to identify patients with similar levels of biomarkers in their blood to assemble a cohort at a similar stage of disease development (the inability to do this has been accepted as a reason for failure in previous clinical trials). Thirdly to test their drug candidates on the patients’ blood samples before the trial to determine if this results in a decrease in the proteins. PharmaKure can then enrol the patients with the advance knowledge that the cohort has similar biomarker levels and that the drugs are likely to work with these patients.
Dr. Farid Khan, CEO of PharmaKure explains, “We will enter human trials faster because our combination of ‘repurposed’ drugs is already approved for use , one as an anti-depressant and the other as an anti-psychotic. As well as knowing that they work on the proteins on the patients’ blood cells, we also know they cross the blood brain barrier. This combination of CDX’s technologies, together with our ground-breaking proprietary formulations, accelerates a promising treatment for these debilitating diseases”.
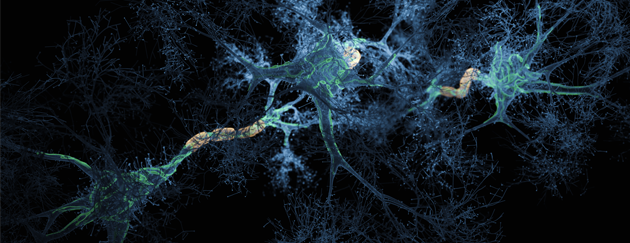
Societies suffer enormous human and financial costs of Alzheimer’s Disease for which there are no cures on the market – only drugs which attempt to alleviate symptoms. Alzheimer’s Disease affects the brain – causing loss in memory and bodily functions, ultimately leading to death. New drugs in clinical trials experience high failure rates in safety and efficacy. In financial terms, Alzheimer’s Disease and Related Dementias (ADRD) currently cost the world economies more money than any other single disease: $1TN globally and growing (source: July 30, 2019; IOS Press). The human cost of such a cruel disease is even more devastating – both to those suffering from this terrible illness and also those who care for them and who see loved ones gradually and desperately disintegrate before their eyes, no longer able to carry out basic functions or to remember their own children.
PharmaKure targets ‘remediation’ of the disease using two re-purposed previously approved drugs. Because the drugs are already approved by regulators, Pharmakure can move quickly to Phase II clinical trials, thus avoiding pre-clinical and Phase I trials which can take years, cost many millions of dollars and give extra steps at which a drug may fail.
Dr. Khan further explains, “We are the first in the world to have demonstrated clinical improvement in a compassionate trial study for Gerstmann–Sträussler–Scheinker syndrome (GSS) – a rare prion disease resembling Alzheimer’s disease– where we managed to partially restore cognitive, memory and mobility functions. With CDX’s technology, we can accelerate the selection of highly suitable candidates for our drug trials and bring the likelihood of approval much closer. No other company has this technology and we think others doing development of Alzheimer’s and related dementias research will want to partner with us to enhance their research. We welcome all who want to solve this terrible disease – come join us!”.
Damian Bond, CEO and Founder of CynapseDx, who will join PharmaKure’s team to focus on the apheresis technology, said “We are proud that our work at CDX has conclusively proven that these toxic misfolded proteins are present in blood and associated with blood cells. Not understanding this has been a critical barrier to developing blood tests for ADRS diseases, which are safer and less painful than collection of Cerebral Spinal Fluid. Blood samples, then become a surrogate for what is happening in the brain and provide a tool to investigate drug candidates that work in blood and hopefully on the plaques and deposits in the brain in the same manner. We think our testing does that. So, while PharmaKure now can focus on using our testing to roll out their drug therapies, I will assume lead responsibility for developing the apheresis technology as a second therapy, using a treatment that is widely available through the UK NHS. I’m pleased to be joining the Pharmakure team and look forward to the dramatic advancement we see in front of us.”
Dr Christopher Stanley, co-director of CynapseDx, will be joining PharmaKure to head the Company’s scientific development effort. Dr Stanley offered these thoughts about this development “I’ve been working closely with Dr Khan for many years. We share the same passion for finding a solution to ADRS diseases and I believe that joining forces, with capital behind us, is all the catalyst we need to create a world-class pipeline of therapies. Our mission is the joint pursuit of a breakthrough in the world of preventive and curative solutions for these terrible diseases.” The pipeline of drugs currently being developed or repurposed by PharmaKure will be enhanced by the formal addition of Dr Stanley to the PharmaKure team and will add momentum and expansion to the Company’s plans to become a significant player in next generation of drug re-purposing treatments for neurological diseases.