Our Science
The PharmaKure solution


Traditional drug discovery aims to find compounds that can interfere in a disease, often by binding to an enzyme or receptor implicated in the disease. The binding alters the target’s function with a beneficial outcome in the disease.
Hundreds of thousands of compounds are tested for target binding through high throughput screening. This approach has typically taken 10-15 years with a cost of about $1.3 billion per successful drug launch. These enormous costs are due to high attrition rates of preclinical candidates because of lack of efficacy or unexpected safety concerns seen during clinical trials.
Screen: PharmaKure’s solution to this development challenge is to screen molecules that have already been through some degree of clinical development in order to discover novel activities in other diseases. This ‘repositioning’ of existing or old drugs for new targets has the following advantages:
• The high risk of failure in transfer from pre-clinical to phase 1 trials is avoided
• The toxicology and side effect profiles of the drugs are known
• This saves years and millions of pounds in development
• Potentially a lower dosage could be prescribed, reducing side effects
• Combinations of drugs may demonstrate improved performance
The drug repositioning or repurposing strategy is not new. It has already led to several blockbuster drugs, for example, Viagra, which was initially intended to treat pulmonary hypertension, but has since been used to treat erectile dysfunction; and Rogaine which was intended to treat high blood pressure has been subsequently repositioned for treatment of hair loss and promotion of hair re-growth. No repurposing program has been applied to Alzheimer’s disease.
Select: PharmaKure’s propriety biomarker assays for Alzheimer’s Disease (ALZmetrix®) and for Parkinson’s Disease (PARKmetrix®) detects the toxic forms of both amyloid-β and α-synuclein, respectively. This allows us to select drugs based on their effect on blood samples.
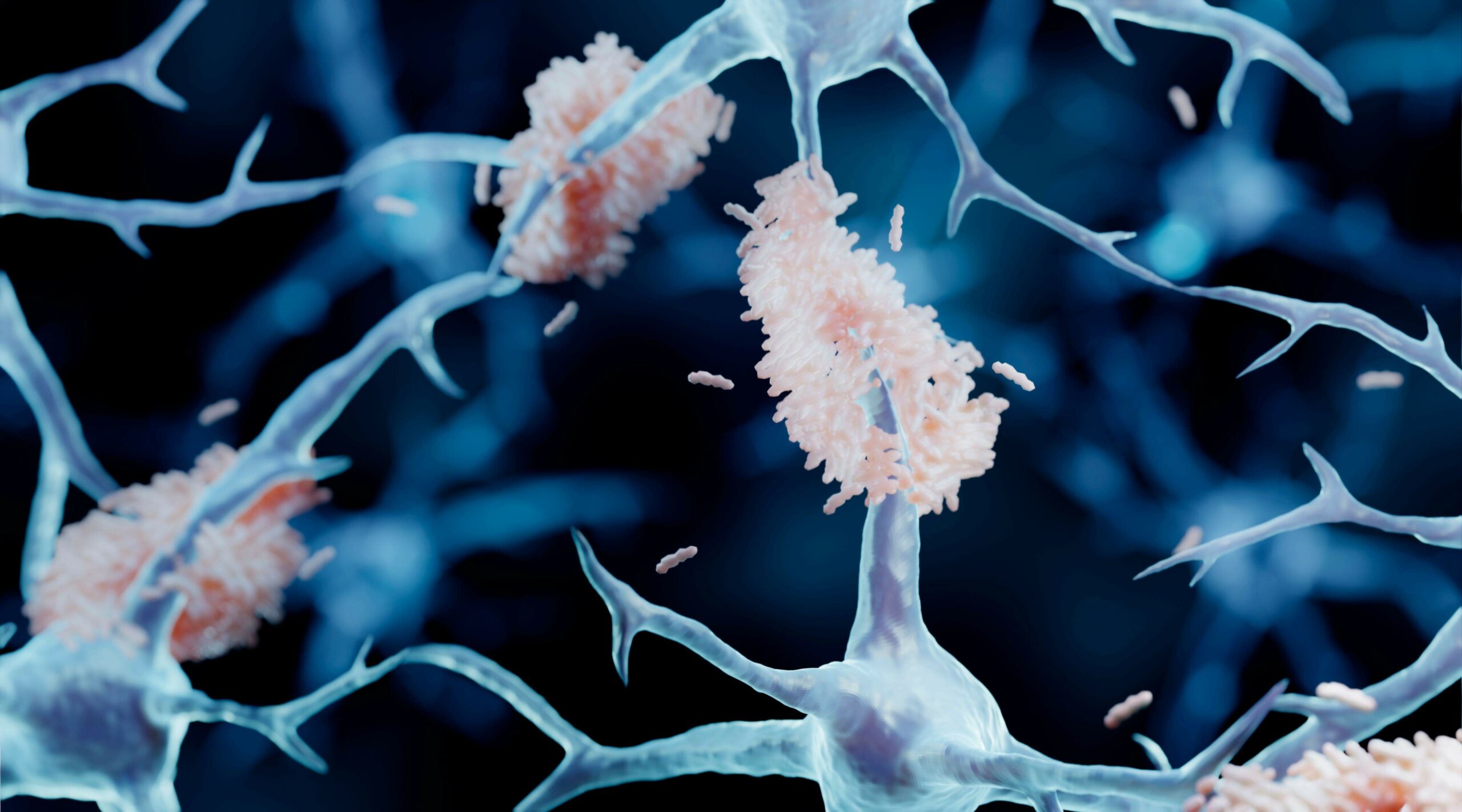
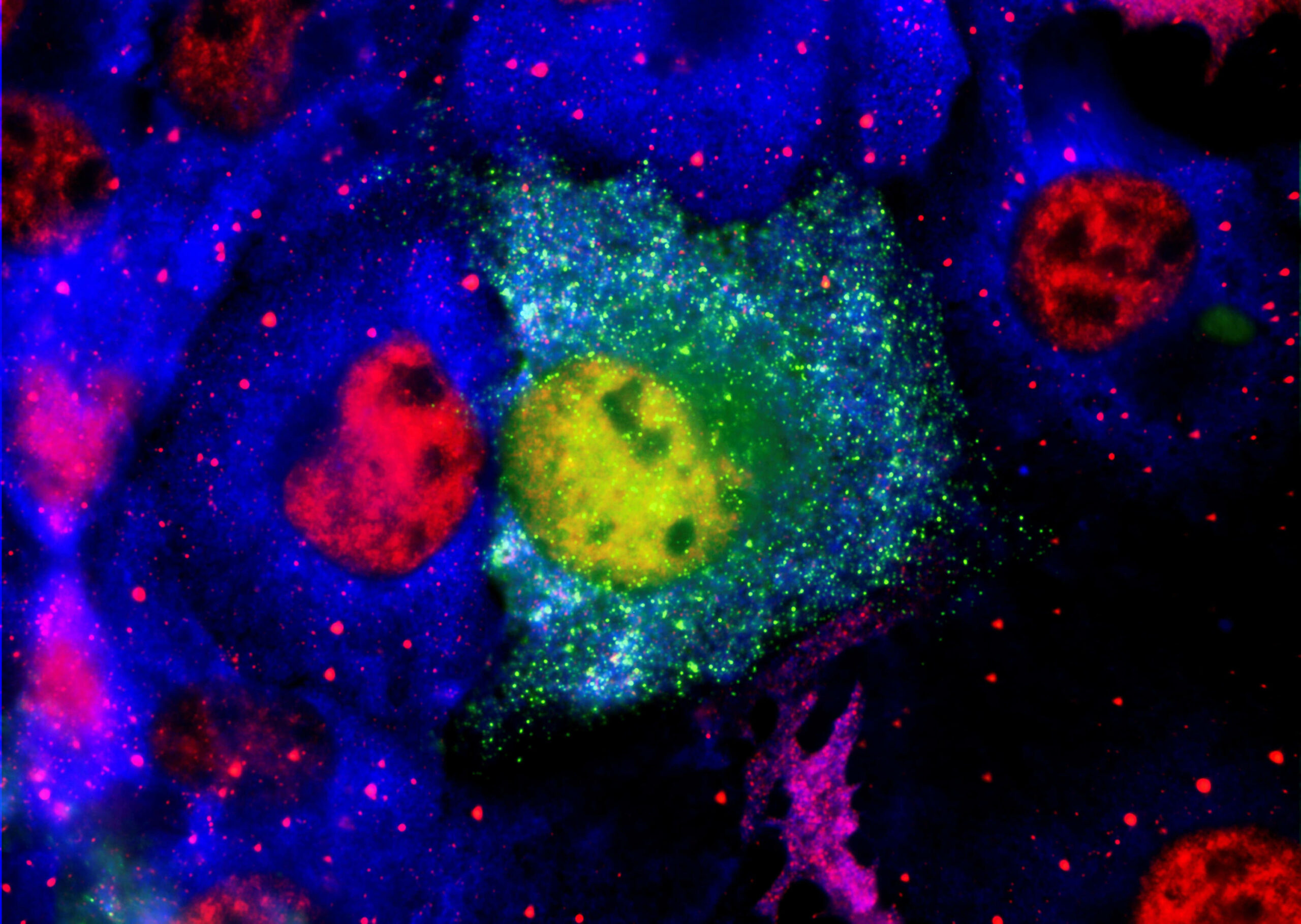
The scientific literature makes it increasingly clear that the toxic forms of these proteins that cause neuronal damage as small soluble aggregates usually termed oligomers. These were thought to be confined to the brain or spinal fluid as investigators could not reliably measure them in blood.
The PharmaKure breakthrough confirmed that these oligomers are present in blood, but attached to blood cells. Previous investigators had missed this as they discarded the blood cells and tested the remaining plasma, where concentrations are very low.
The healthy form of these proteins are thought to be monomers or single proteins. Oligomers form when they misfold. They become seeds that can cause more proteins to misfold and grow into oligomers.
A key strategy for drug or antibody treatment of Alzheimer’s disease has been plaque busting i.e. breaking apart the amyloid-β plaques in the brain. If the liberated amyloid-β remains, it could seed more oligomers and speed up the disease progression. In fact recent publications suggest that plaque formation may be a defence mechanism to mop up these toxic oligomers.
• Through use of the ALZmetrix® and PARKmetrix® assays, PharmaKure has a unique technology to rapidly and inexpensively test a drug, or drug combinations in the laboratory for their ability to break down oligomers and prevent them from reforming. If the drugs work the levels of oligomeric amyloid-β in a patient’s blood sample will reduce and not build back up on storage.
• They can also help select patients for clinical trials who have demonstrated a positive response on their blood to the drug candidates.
• They can also select patients for inclusion into cohorts based on similar blood oligomer levels. A critical factor in failure in clinical trials for Alzheimer’s disease has been an inability to select patients at the same stage of disease. These biomarkers offer a new way to help minimise this impact.
PharmaKure’s approach
Develop current and future products
Bring cost effective treatments and cures to the market quickly
Improve patients’ quality of life
Validate: through Clinical Trials
Phase 1 trials have already been completed on these repurposed drugs and do not need repeating.
It is likely that the dosage for a re-purposed drug or combination may be different to the original disease. Trials therefore start with a phase 2a trial to investigate dose escalation. For PharmaKure’s lead drug candidate, this involves 40 patients over a 3 week trial to identify the optimal dosage.
The selected dose will then proceed into a phase 2b trial involving 200 patients over 12 months. In clinical trial terms this is very short and consequently relatively inexpensive.
During the 12 months trial patients will visit the clinic where in addition to the cognitive, pharmacodynamic and safety assessments that are part of any Alzheimer’s disease drug trial, PharmaKure have ability to measure levels of oligomer amyloid-β as an additional guide to disease progression.
• Shorter trials
• Less expensive trials
• New assessment of disease progression using blood biomarkers.